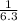Chemistry, 24.03.2020 23:12 ShelleyPSeliger
For the chemical equation SO 2 ( g ) + NO 2 ( g ) − ⇀ ↽ − SO 3 ( g ) + NO ( g ) the equilibrium constant at a certain temperature is 3.10 . At this temperature, calculate the number of moles of NO 2 ( g ) that must be added to 2.30 mol SO 2 ( g ) in order to form 1.00 mol SO 3 ( g ) at equilibrium.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
For the chemical equation SO 2 ( g ) + NO 2 ( g ) − ⇀ ↽ − SO 3 ( g ) + NO ( g ) the equilibrium cons...
Questions

Mathematics, 24.02.2020 22:25





Mathematics, 24.02.2020 22:25



Mathematics, 24.02.2020 22:25



English, 24.02.2020 22:26


Health, 24.02.2020 22:26


Mathematics, 24.02.2020 22:26

Geography, 24.02.2020 22:26



 =
= ![\frac{[SO_{3} ][NO]}{[SO_{2}][NO_{2} ]}](/tpl/images/0561/8606/bc0fb.png)
![\frac{(1.00)(1.00)}{(2.30) [NO_{2} ]}](/tpl/images/0561/8606/3f85b.png) At equilibrium [SO₃] = [NO]
At equilibrium [SO₃] = [NO]