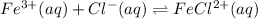Chemistry, 24.03.2020 23:08 tstaples02
Use Le Chatelier’s principle to predict how the equilibrium concentration of the FeCl2+ ion will change when an aqueous solution of silver (I) nitrate, AgNO3, is added to the exothermic reaction below. Fe3+(aq) + Cl-(aq) → FeCl2+(aq)

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Use Le Chatelier’s principle to predict how the equilibrium concentration of the FeCl2+ ion will cha...
Questions



Mathematics, 21.02.2021 20:30

Mathematics, 21.02.2021 20:30

Biology, 21.02.2021 20:30

English, 21.02.2021 20:30


Mathematics, 21.02.2021 20:30

Physics, 21.02.2021 20:30


Spanish, 21.02.2021 20:30

Mathematics, 21.02.2021 20:30




Spanish, 21.02.2021 20:30


Mathematics, 21.02.2021 20:30

Geography, 21.02.2021 20:30

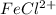 will decrease.
will decrease.