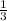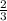Chemistry, 25.03.2020 01:21 FavvBella84
The total pressure for a mixture of N2O4 and NO2 is 1.5atm. If Kp = 7.1 at 25°C, calculate the partial pressure of each gas in the mixture. (HINT 1.5 = 2pNO2 + pN2O4) 2NO2 (g) = N2O4 (g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 19:00
Imagine that a new planet is discovered with two moons of equal mass: moon a and moon b. the mass of the new planet is greater than the combined mass of its moons. moon a is farther away from the new planet than moon b. what is the planet's gravitational pull on moon a compared to the planet's gravitational pull on moon b? the planet's gravity repels moon a with a greater force than it repels moon b, which is why moon a is farther away. the gravitational pull on moon b is greater than on moon a because moon b is closer to the new planet than moon a. the gravitational pull on moon b is greater than on moon a because moon b is farther away from the new planet than moon a. the gravitational pull on moon a is the same as the gravitational pull on moon b because distance does not affect the planet's gravity.
Answers: 1

Chemistry, 23.06.2019 12:30
)a children’s liquid cold medicine has a density of 1.23 g/ml. if a child is to take 2.5 tsp in a dose, what is the mass in grams of this dose? (1 tsp = 5 ml)
Answers: 1
You know the right answer?
The total pressure for a mixture of N2O4 and NO2 is 1.5atm. If Kp = 7.1 at 25°C, calculate the parti...
Questions




Mathematics, 18.01.2020 02:31

Mathematics, 18.01.2020 02:31


Health, 18.01.2020 02:31

Mathematics, 18.01.2020 02:31



Mathematics, 18.01.2020 02:31

Mathematics, 18.01.2020 02:31

Geography, 18.01.2020 02:31





Mathematics, 18.01.2020 02:31


Mathematics, 18.01.2020 02:31

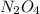 : 0.5 atm and
: 0.5 atm and 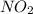 : 1 atm
: 1 atm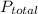 = 1.5 atm
= 1.5 atm