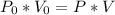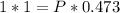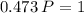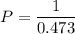Chemistry, 25.03.2020 02:10 pineappleliyah3
1.00 L of a gas at STP is compressed to 473mL. What is the new pressure of gas?

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 18:10
Which is an aspect of the kinetic-molecular theory and can be used to explain the behavior of plasmas? particle spacing can allow a very high density. particle kinetic energy is independent of temperature. particles vibrate quickly in stationary positions. particles exchange energy through elastic collisions.
Answers: 2

Chemistry, 23.06.2019 19:30
Why does 4.03/0.0000035 = 1.2 x 106, instead of a different number of significant figures?
Answers: 1

Chemistry, 23.06.2019 21:00
Areaction in which a, b, and c react to form products is zero order in a, one-half order in b, and second order in c. by what factor does the reaction rate change if the concentration of a is doubled? 1 by what factor does the reaction rate change if the concentration of b is doubled? 1.4 by what factor does the reaction rate change if the concentration of c is doubled? 4
Answers: 1

Chemistry, 23.06.2019 23:00
2.50 mol nocl was placed in a 2.50 l reaction vessel at 400ºc. after equilibrium was established, it was found that 28% of the nocl had dissociated according to the equation 2nocl(g) picture 2no(g) + cl2(g). calculate the equilibrium constant, kc, for the reaction.
Answers: 2
You know the right answer?
1.00 L of a gas at STP is compressed to 473mL. What is the new pressure of gas?...
Questions


Computers and Technology, 28.05.2021 20:30




Biology, 28.05.2021 20:30

Mathematics, 28.05.2021 20:30


Mathematics, 28.05.2021 20:30



Biology, 28.05.2021 20:30



Computers and Technology, 28.05.2021 20:30




Mathematics, 28.05.2021 20:30