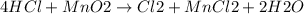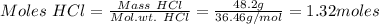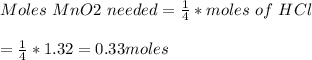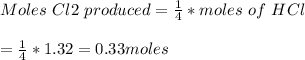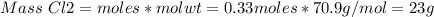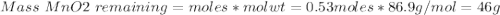Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
If 0.86 mole of mno2 and 48.2 g of hcl react, which reagent will be used up first? how many grams o...
Questions


Computers and Technology, 14.05.2021 01:30

Chemistry, 14.05.2021 01:30



Mathematics, 14.05.2021 01:30

Mathematics, 14.05.2021 01:30



Mathematics, 14.05.2021 01:30

Mathematics, 14.05.2021 01:30

Social Studies, 14.05.2021 01:30

Mathematics, 14.05.2021 01:30

Mathematics, 14.05.2021 01:30

Mathematics, 14.05.2021 01:30

Mathematics, 14.05.2021 01:30


Mathematics, 14.05.2021 01:30


Mathematics, 14.05.2021 01:30