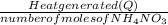Chemistry, 25.03.2020 02:03 AkramMasoud
Calculate the change in enthalpy for the reaction. (Use 1.0 g/mL as the density of the solution and 4.18 J/(g⋅∘C) as the specific heat capacity.) Express the enthalpy change in kilojoules per mole to two significant figures. ΔHrxn = nothing kJ/mol

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1
You know the right answer?
Calculate the change in enthalpy for the reaction. (Use 1.0 g/mL as the density of the solution and...
Questions



Mathematics, 11.05.2021 06:40

Mathematics, 11.05.2021 06:40

Mathematics, 11.05.2021 06:40




Mathematics, 11.05.2021 06:40

Mathematics, 11.05.2021 06:40

Mathematics, 11.05.2021 06:40

Mathematics, 11.05.2021 06:40


Mathematics, 11.05.2021 06:40




Mathematics, 11.05.2021 06:40


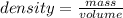

 =
=