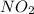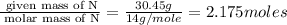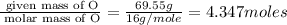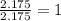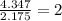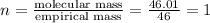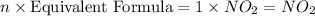Chemistry, 25.03.2020 02:00 AlaskaAirlines
A compound is found to contain 30.45 % nitrogen and 69.55 % oxygen by mass. To answer the question, enter the elements in the order presented above. QUESTION 1: The empirical formula for this compound is . QUESTION 2: The molar mass for this compound is 46.01 g/mol. The molecular formula for this compound is .

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 23.06.2019 10:30
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2


Chemistry, 23.06.2019 12:30
15) a substance used in manufacturing gasoline consists of finely divided platinum supported on an inert solid. suppose that the platinum is formed by the high temperature reaction between platinum (iv) oxide and hydrogen gas. the other product is water. a) write and balance the equation b) how many grams of hydrogen are needed to produce 1.0 g of platinum metal? c) how many moles of water are produced at the same time? how many grams? ( show work, .)
Answers: 1
You know the right answer?
A compound is found to contain 30.45 % nitrogen and 69.55 % oxygen by mass. To answer the question,...
Questions

Chemistry, 09.12.2020 06:20


Social Studies, 09.12.2020 06:20

Biology, 09.12.2020 06:20


Biology, 09.12.2020 06:20

Mathematics, 09.12.2020 06:20

History, 09.12.2020 06:20




Social Studies, 09.12.2020 06:20


Mathematics, 09.12.2020 06:20

Computers and Technology, 09.12.2020 06:20

Mathematics, 09.12.2020 06:20

Mathematics, 09.12.2020 06:20

History, 09.12.2020 06:20