Chemistry, 25.03.2020 03:05 JakkuZakku9436
Octane (C8H18) undergoes combustion according to the following thermochemical equation. 2C8H18(l) + 25O2(g) → 16CO2(g) + 18H2O(l) ΔH°rxn = –1.0940 × 104 kJ/mol What is the standard enthalpy of formation of liquid octane? ΔH°f(CO2(g)) = –393.5 kJ/mol and ΔH°f(H2O(l)) = –285.8 kJ/mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1


You know the right answer?
Octane (C8H18) undergoes combustion according to the following thermochemical equation. 2C8H18(l) +...
Questions

Mathematics, 25.08.2021 14:00




Business, 25.08.2021 14:00


English, 25.08.2021 14:00

Biology, 25.08.2021 14:00

Mathematics, 25.08.2021 14:00

Mathematics, 25.08.2021 14:00

Mathematics, 25.08.2021 14:00


Mathematics, 25.08.2021 14:00



Geography, 25.08.2021 14:00


Mathematics, 25.08.2021 14:00

Chemistry, 25.08.2021 14:00

Biology, 25.08.2021 14:00

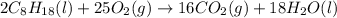
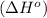 .
.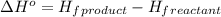
![\Delta H^o=[n_{O_2}\times \Delta H_f^0_{(O_2)}+n_{H_2O}\times \Delta H_f^0_{(H_2O)}]-[n_{C_8H_{18}}\times \Delta H_f^0_{(C_8H_{18})+n_{O_2}\times \Delta H_f^0_{(O_2)}]](/tpl/images/0562/3120/bdc44.png)
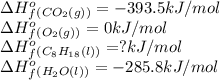
![-1.0940\times 10^4=[(16\times -393.5)+(18\times -285.8)]-[(25\times 0)+(2\times \Delat H_f{C_8H_{18}(l)}]](/tpl/images/0562/3120/9e959.png)