
Predict whether the equilibria
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° = -90 kJ
II) Ni(s) + 4 CO(g) = Ni(CO)4(8),
AH° = -161 kJ
will shift toward products or reactants with a
temperature increase.
1. I shifts toward reactants and II shifts
toward products.
2. Both I and II shift toward products.
3. Unable to determine
4. Both I and II shift toward reactants.
5. I shifts toward products and II shifts to-
ward reactants.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

You know the right answer?
Predict whether the equilibria
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° =...
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° =...
Questions

Arts, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

Advanced Placement (AP), 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

Business, 20.05.2021 01:00

Biology, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

Business, 20.05.2021 01:00

Spanish, 20.05.2021 01:00


English, 20.05.2021 01:00


Mathematics, 20.05.2021 01:00

English, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

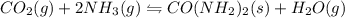 ,ΔH° = -90 kJ
,ΔH° = -90 kJ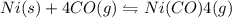 ,ΔH° = -161 kJ
,ΔH° = -161 kJ


