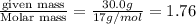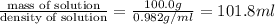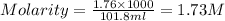Chemistry, 25.03.2020 03:47 haileywatkins
3. Calculate the molarity of an NH3 aqueous solution made up of 30.0 g of NH3 and 70.0 g of water at 25 °C. The density of water at that temperature is 0.982 g mL−1. M(NH3) = 17.034 g mol−1 A. 17.3 mol L−1 B. 24.7 mol L−1 C. 5.78 × 10−2 mol L−1 D. 0.578 mol L−1 E. 1.73 mol L−1

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 23.06.2019 09:30
People who practice which of the following diets may run the risk of not getting enough iron. a. gluten free or vegan diet b. diet for managing diabetes c. vegan diet d. gluten free diet
Answers: 2

Chemistry, 23.06.2019 14:00
[07.06] which of the following chemical reactions is an oxidation-reduction reaction? (2 points) wo3 + 3h2 yields w + 3h2o kno3 + licl yields lino3 + kcl caso4 + 2nacl yields na2so4 + cacl2 mg(no3)2 + 2hbr yields mgbr2 + 2hno3
Answers: 1
You know the right answer?
3. Calculate the molarity of an NH3 aqueous solution made up of 30.0 g of NH3 and 70.0 g of water at...
Questions

History, 26.06.2019 09:00

Mathematics, 26.06.2019 09:00

History, 26.06.2019 09:00

Chemistry, 26.06.2019 09:00

History, 26.06.2019 09:00



Biology, 26.06.2019 09:00

Mathematics, 26.06.2019 09:00

Mathematics, 26.06.2019 09:00

Mathematics, 26.06.2019 09:00

Mathematics, 26.06.2019 09:00

Social Studies, 26.06.2019 09:00

Biology, 26.06.2019 09:00

Health, 26.06.2019 09:00

Biology, 26.06.2019 09:00


History, 26.06.2019 09:00


Mathematics, 26.06.2019 09:00


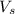 = volume of solution in ml
= volume of solution in ml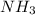 (solute) =
(solute) =