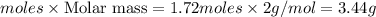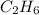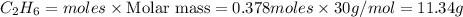Chemistry, 25.03.2020 05:40 queenliz855
For the following reaction, 4.21 grams of hydrogen gas are allowed to react with 10.6 grams of ethylene (C2H4) . hydrogen(g) + ethylene (C2H4)(g) ethane (C2H6)(g) What is the maximum mass of ethane (C2H6) that can be formed? grams What is the FORMULA for the limiting reagent? C2H6 What mass of the excess reagent remains after the reaction is complete? grams

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
For the following reaction, 4.21 grams of hydrogen gas are allowed to react with 10.6 grams of ethyl...
Questions

Mathematics, 24.10.2021 08:30

SAT, 24.10.2021 08:30

SAT, 24.10.2021 08:30

Physics, 24.10.2021 08:30



Mathematics, 24.10.2021 08:30



Physics, 24.10.2021 08:30



Geography, 24.10.2021 08:30


Engineering, 24.10.2021 08:30

Social Studies, 24.10.2021 08:30



Biology, 24.10.2021 08:30

English, 24.10.2021 08:30

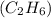 can be formed
can be formed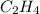 is the limiting reagent
is the limiting reagent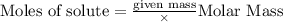
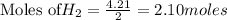
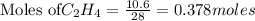
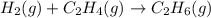
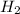
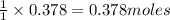 of
of