Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 21.06.2019 22:30
Monkeys and bats have similar bone structure in their forelimbs. however, monkeys have longer forelimbs to use for climbing and swinging in trees. bats have shorter forelimbs to use for flight. which term best describes how monkey and bat forelimbs are related to each other? a. homologous b. embryonic c. analogous d. vestigial
Answers: 1

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1
You know the right answer?
Titanium reacts with iodine to form titanium(III) iodide, emitting heat, via the following reaction:...
Questions




Computers and Technology, 24.02.2020 17:01


Mathematics, 24.02.2020 17:01

Chemistry, 24.02.2020 17:01



Mathematics, 24.02.2020 17:01










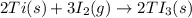

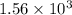 kJ of heat is released by =
kJ of heat is released by = 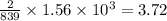 moles of titanium
moles of titanium