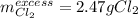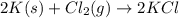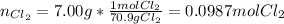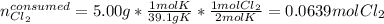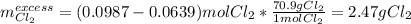Chemistry, 25.03.2020 04:55 arizola757
Potassium chloride is used as a substitute for sodium chloride for individuals with high blood pressure. Identify the limiting reactant and determine the mass of the excess reactant remaining when 7.00 g of chlorine gas reacts with 5.00 g of potassium to form potassium chloride.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 23:30
Which question cannot be answered scientifically? a. how many particles do two gases at the same temperature and pressure contain? b. what happens to a gas at standard temperature and pressure? c. how does a gas react when heated to 100 degrees celsius? d. what happens to a sample of gas at absolute zero?
Answers: 2

Chemistry, 24.06.2019 00:30
The ksp of srso4 is 3.2 × 10–7. what is the equilibrium concentration of sulfate ion in a 1.0-l solution of strontium sulfate to which 0.10 mol of sr(ch3co2)2 has been added? a. 3.2 × 10–8m b. 3.2 × 10–6m c. 5.7 × 10–4m d. 8.0 × 10–4m
Answers: 1
You know the right answer?
Potassium chloride is used as a substitute for sodium chloride for individuals with high blood press...
Questions






History, 01.07.2019 09:30


Biology, 01.07.2019 09:30

Mathematics, 01.07.2019 09:30

English, 01.07.2019 09:30

Biology, 01.07.2019 09:30


Mathematics, 01.07.2019 09:30

Mathematics, 01.07.2019 09:30




Spanish, 01.07.2019 09:30