
Chemistry, 25.03.2020 05:07 dontcareanyonemo
Without consulting any tables, arrange the following substances in order and explain your choice of order: (a) Mg21, Ar, Br2, Ca21 in order of increasing radius (b) Na, Na1, O, Ne in order of increasing ionization energy (c) H, F, Al, O in order of increasing electronegativity

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
Without consulting any tables, arrange the following substances in order and explain your choice of...
Questions



Chemistry, 25.01.2020 09:31

History, 25.01.2020 09:31

Biology, 25.01.2020 09:31




Health, 25.01.2020 09:31

History, 25.01.2020 09:31

Mathematics, 25.01.2020 09:31



Mathematics, 25.01.2020 09:31


Mathematics, 25.01.2020 09:31

Social Studies, 25.01.2020 09:31



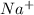 = Ne
= Ne

