
Chemistry, 25.03.2020 04:59 levelebeasley1
Suppose a 1.0 L solution contains 0.020 M in Cu(NO3)2, then 0.40 moles of NH3 are added. Assuming no change in volume, what is the concentration of Cu2+ ions in the solution after the addition of NH3? The Kf for Cu(NH3)42+ is 1.7x1013.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
Suppose a 1.0 L solution contains 0.020 M in Cu(NO3)2, then 0.40 moles of NH3 are added. Assuming no...
Questions










Biology, 16.07.2019 02:20

Mathematics, 16.07.2019 02:20

English, 16.07.2019 02:20




History, 16.07.2019 02:20

Computers and Technology, 16.07.2019 02:20


Mathematics, 16.07.2019 02:20

History, 16.07.2019 02:20

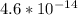 M
M![Cu^{2+} = [Cu(NO_3)_2]](/tpl/images/0562/4161/18067.png) = 0.020 M
= 0.020 M![Cu^{2+}+4NH_3_{aq} \rightleftharpoons [Cu(NH_3)_4]^{2+}_{(aq)}](/tpl/images/0562/4161/56ee7.png)
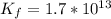
![K_f } = \frac{[Cu(NH_3)_4]^{2+}}{[Cu^{2+}][NH_3]^4}](/tpl/images/0562/4161/1ed4c.png)
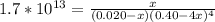
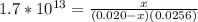
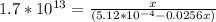
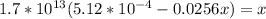
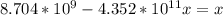
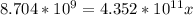
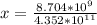
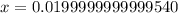
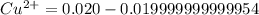
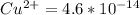 M
M 

