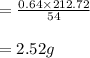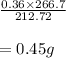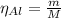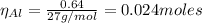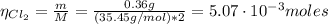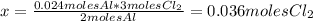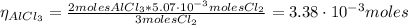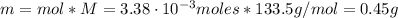Chemistry, 25.03.2020 05:47 ruchierosanp1n3qw
The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s) + 3Cl2(g) → 2AlCl3(s) Assume that 0.64 g Al is mixed with 0.36 g Cl2. (a) What is the limiting reactant? Al Cl2 (b) What is the maximum amount of AlCl3, in grams, that can be produced?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:40
Which are causes of mechanical weathering? check all that apply.oacid raino plant growtho animal actionso carbon dioxideo pressure release
Answers: 1

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1
You know the right answer?
The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s) + 3Cl2(g) → 2AlC...
Questions



Biology, 29.01.2021 23:00

Social Studies, 29.01.2021 23:00


Mathematics, 29.01.2021 23:00


Arts, 29.01.2021 23:00

English, 29.01.2021 23:00


Arts, 29.01.2021 23:00









Mathematics, 29.01.2021 23:00