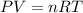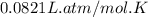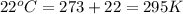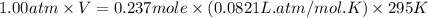Chemistry, 25.03.2020 05:36 lelseymota123
Automobile airbags contain solid sodium azide, NaN 3 , that reacts to produce nitrogen gas when heated, thus inflating the bag. 2 NaN 3 ( s ) ⟶ 2 Na ( s ) + 3 N 2 ( g ) Calculate the value of work, w , for the system if 10.3 g NaN 3 reacts completely at 1.00 atm and 22 ∘ C.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1

Chemistry, 23.06.2019 11:40
Which of the following observations indicates that an atom has neutrons? some uncharged particles are scattered by a beryllium atom when it hits a gold foil. some uncharged particles bounce back from a gold foil when it is bombarded with alpha particles. a radiation consisting of uncharged particles is emitted when alpha particles strike beryllium atoms. a radiation which attracts electrons is produced when a beryllium atom is bombarded with alpha particles.
Answers: 2

Chemistry, 23.06.2019 14:30
Among the elements of the main group the first ionization energy increases
Answers: 3
You know the right answer?
Automobile airbags contain solid sodium azide, NaN 3 , that reacts to produce nitrogen gas when heat...
Questions

History, 29.10.2020 16:50



Arts, 29.10.2020 16:50


Mathematics, 29.10.2020 16:50







Mathematics, 29.10.2020 16:50

World Languages, 29.10.2020 16:50

Mathematics, 29.10.2020 16:50

Physics, 29.10.2020 16:50


Health, 29.10.2020 16:50


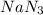
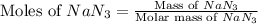
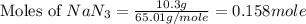
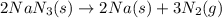
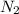
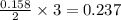 moles of
moles of