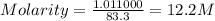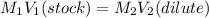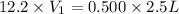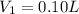Chemistry, 25.03.2020 05:31 wallsdeandre25521
Hydrochloric acid is usually purchased in concentrated form with a 37.0% HCl concentration by mass and a density of 1.20 g / mL. How much of the concentrated stock solution in milliliters should you use to make 2.5 L of 0.500 M HCl

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:30
How many joules of heat are absorbed to raise the temperature of 650 grams of water from 5.00c to it's boiling point, 100c
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3
You know the right answer?
Hydrochloric acid is usually purchased in concentrated form with a 37.0% HCl concentration by mass a...
Questions

Mathematics, 14.10.2020 18:01



Mathematics, 14.10.2020 18:01

English, 14.10.2020 18:01



Mathematics, 14.10.2020 18:01

Chemistry, 14.10.2020 18:01

Mathematics, 14.10.2020 18:01


Mathematics, 14.10.2020 18:01


Mathematics, 14.10.2020 18:01


History, 14.10.2020 18:01


Mathematics, 14.10.2020 18:01


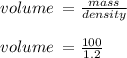
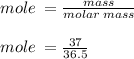
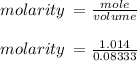
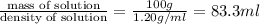
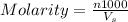
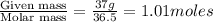
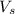 = volume of solution in ml = 83.3 ml
= volume of solution in ml = 83.3 ml