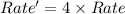Chemistry, 25.03.2020 07:01 mamas4539p79bw7
The reaction X + 2M → products has been found to have the rate law, rate = k[X] [M]2 . While holding the concentration of M constant, the concentration of X is increased from x to 4x. Predict by what factor the rate of reaction increases.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1


Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
The reaction X + 2M → products has been found to have the rate law, rate = k[X] [M]2 . While holding...
Questions

Mathematics, 11.11.2019 23:31



Computers and Technology, 11.11.2019 23:31

Social Studies, 11.11.2019 23:31

Mathematics, 11.11.2019 23:31

Mathematics, 11.11.2019 23:31

Biology, 11.11.2019 23:31


Biology, 11.11.2019 23:31



Mathematics, 11.11.2019 23:31




Mathematics, 11.11.2019 23:31



Physics, 11.11.2019 23:31

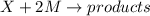
![Rate=k[X][M]^2](/tpl/images/0562/8462/02d02.png)
![Rate'=k[4X]^1[M]^2](/tpl/images/0562/8462/66381.png)
![Rate'=k[4]^1[X]^1[M]^2](/tpl/images/0562/8462/31177.png)