
Chemistry, 25.03.2020 06:43 letyourmemesbedreams
Consider the reaction: P(s) + 5/2 Cl2(g)PCl5(g) Write the equilibrium constant for this reaction in terms of the equilibrium constants, Ka and Kb, for reactions a and b below: a.) P(s) + 3/2 Cl2(g) PCl3(g) Ka b.) PCl3(g) + Cl2(g) PCl5(g) Kb

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

You know the right answer?
Consider the reaction: P(s) + 5/2 Cl2(g)PCl5(g) Write the equilibrium constant for this reaction in...
Questions

Mathematics, 23.05.2020 23:00




Mathematics, 23.05.2020 23:00


Mathematics, 23.05.2020 23:00

Mathematics, 23.05.2020 23:00

English, 23.05.2020 23:00

Biology, 23.05.2020 23:00


Mathematics, 23.05.2020 23:00








Mathematics, 23.05.2020 23:01


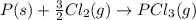
![K_a=\frac{[PCl_3]}{[Cl_2]^{\frac{3}{2}}}](/tpl/images/0562/7849/72fe4.png)
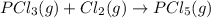
![K_b=\frac{[PCl_5]}{[Cl_2]\times [PCl_3]}](/tpl/images/0562/7849/4a569.png)
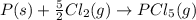
![K_c=\frac{[PCl_5]}{[Cl_2]^\frac{5}{2}}](/tpl/images/0562/7849/aecd1.png)
![K_c=K_a\times K_b=\frac{[PCl_3]}{[Cl_2]^{\frac{3}{2}}}\times \frac{[PCl_5]}{[Cl_2]\times [PCl_3]}](/tpl/images/0562/7849/835c8.png)


