
Chemistry, 25.03.2020 06:21 miacespedes
Consider the following reaction 2 N2O(g) → 2 N2(g) + O2(g) rate = k [N2O] . For an initial concentration of N2O of 0.50 M, calculate the concentration of N2O remaining after 2.0 min if k = 3.4 × 10−3 s −1 . 1. 0.17 M 2. 0.50 M 3. 0.55 M 4. 0.33 M 5. 0.66 M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 13:30
What is matter? a. anything that has mass and takes up space b. something that has volume and takes up space. c. things that have energy and take up space d. things that take up space but don't have mass
Answers: 2
You know the right answer?
Consider the following reaction 2 N2O(g) → 2 N2(g) + O2(g) rate = k [N2O] . For an initial concentra...
Questions



Mathematics, 22.01.2021 21:00

Arts, 22.01.2021 21:00




Spanish, 22.01.2021 21:00

Mathematics, 22.01.2021 21:00

Mathematics, 22.01.2021 21:00

Mathematics, 22.01.2021 21:00


Mathematics, 22.01.2021 21:00




Social Studies, 22.01.2021 21:00


Mathematics, 22.01.2021 21:00

English, 22.01.2021 21:00

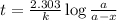
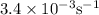
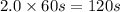
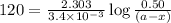
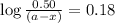
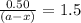
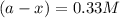
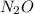 remaining after 2.0 min is 0.33 M
remaining after 2.0 min is 0.33 M

