Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1

Chemistry, 23.06.2019 14:30
When carbon disulfide is formed from its elements, heat is absorbed. calculate the amount of heat in (kj) absorbed when 5.66g of carbon disulfide is formed
Answers: 3

Chemistry, 23.06.2019 18:10
A1 forms when an acid is neutralized by a base. 1. salts can be neutral, or in solutions. salts of 2. strong acid–strong base reactions produce solutions with 3. water. salts formed from the neutralization of weak acids or weak 4. bases water. they produce solutions that are acidic or . basic. for example, the ph of a solution at the equivalence point is . greater than for a acid titration. solutions that resist changes in ph are called solutions. the buffer is the amount of acid or base that can be added to a buffer without changing the ph greatly.
Answers: 1
You know the right answer?
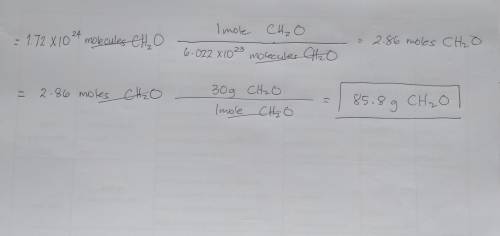
Calculate the mass in grams of 1.72×10^24 molecules of formaldehyde. The chemical formula for formal...
Questions

Advanced Placement (AP), 25.06.2021 08:40


Social Studies, 25.06.2021 08:40

Business, 25.06.2021 08:40

Mathematics, 25.06.2021 08:40










Spanish, 25.06.2021 08:50


English, 25.06.2021 08:50

English, 25.06.2021 08:50

History, 25.06.2021 08:50

Social Studies, 25.06.2021 08:50