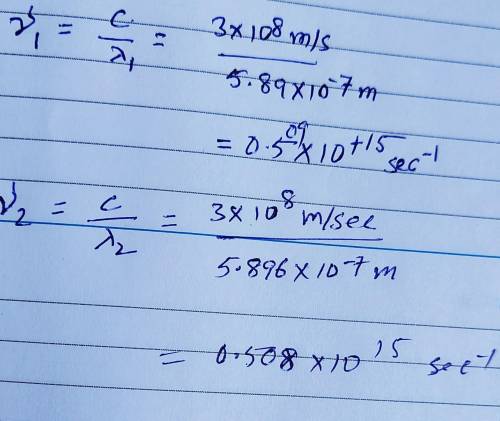Sodium vapor lamps are used to illuminate streets and highways. The very bright light emitted by these lamps is actually due to two closely spaced emission lines in the visible region of the electromagnetic spectrum. One of these lines has a wavelength of 5.890 X 10⁻⁷ m, and the other line has a wavelength of 5.896 X 10⁻⁷ m. A) What are the wavelengths of these radiations in centimeters? B) Calculate the frequencies of these radiations. Show work please

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2


Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1

Chemistry, 23.06.2019 14:00
Ineed in question 5and6 in science the subject is science
Answers: 1
You know the right answer?
Sodium vapor lamps are used to illuminate streets and highways. The very bright light emitted by the...
Questions


Mathematics, 24.05.2021 20:20

Chemistry, 24.05.2021 20:20



Mathematics, 24.05.2021 20:20


English, 24.05.2021 20:20

Health, 24.05.2021 20:20

Mathematics, 24.05.2021 20:20

English, 24.05.2021 20:20


History, 24.05.2021 20:20

Chemistry, 24.05.2021 20:20

Health, 24.05.2021 20:20

Mathematics, 24.05.2021 20:20

Mathematics, 24.05.2021 20:20


Law, 24.05.2021 20:20

English, 24.05.2021 20:20