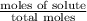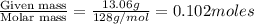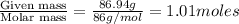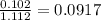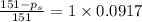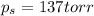Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
A solution contains naphthalene (C10H8) dissolved in hexane (C6H14) at a concentration of 13.06% nap...
Questions


Mathematics, 06.05.2020 08:40


Mathematics, 06.05.2020 08:40

History, 06.05.2020 08:40


Mathematics, 06.05.2020 08:40

English, 06.05.2020 08:40

Physics, 06.05.2020 08:41

English, 06.05.2020 08:41

Mathematics, 06.05.2020 08:41



Advanced Placement (AP), 06.05.2020 08:41



History, 06.05.2020 08:41



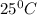 is 137 torr
is 137 torr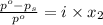
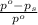 = relative lowering in vapor pressure
= relative lowering in vapor pressure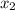 = mole fraction of solute =
= mole fraction of solute =