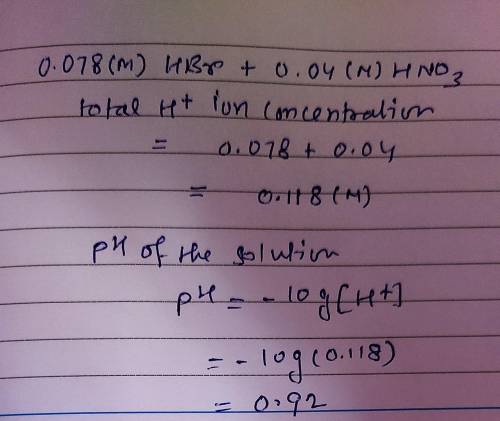Chemistry, 25.03.2020 17:31 keananashville
Determine the ph of an aqueous solution that is 0.078 m in hbr and 0.040 m in hno3.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2


Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
Determine the ph of an aqueous solution that is 0.078 m in hbr and 0.040 m in hno3....
Questions





Mathematics, 13.10.2021 01:40


Health, 13.10.2021 01:40

Biology, 13.10.2021 01:40

Chemistry, 13.10.2021 01:40



English, 13.10.2021 01:40

Biology, 13.10.2021 01:40

Spanish, 13.10.2021 01:40

Biology, 13.10.2021 01:40


Mathematics, 13.10.2021 01:40