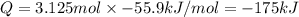125 grams of solid sodium hydroxide is combined with an excess of aqueous hydrochloric acid. The heat of neutralization for the formation of water -55.9 kJ/mole. The heat of solvation (it might be called the heat of solution) for sodium hydroxide is 41.0 kJ/mole What quantity of heat is released?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2
You know the right answer?
125 grams of solid sodium hydroxide is combined with an excess of aqueous hydrochloric acid. The hea...
Questions

English, 29.10.2020 04:30

Social Studies, 29.10.2020 04:30

Mathematics, 29.10.2020 04:30

Biology, 29.10.2020 04:30


Advanced Placement (AP), 29.10.2020 04:30


Mathematics, 29.10.2020 04:30



History, 29.10.2020 04:30

Physics, 29.10.2020 04:30




English, 29.10.2020 04:30


History, 29.10.2020 04:30




 of water
of water