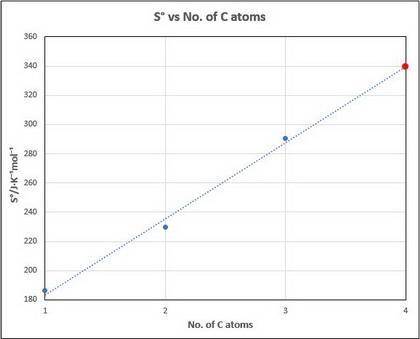
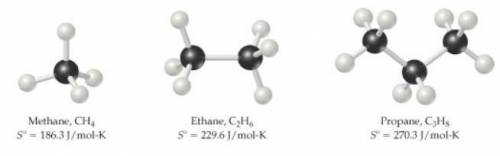
What might you expect for the value of S∘ (entropy) for butane, C4H10?
Entropies provided (Hin...

Chemistry, 25.03.2020 20:08 shaylawaldo11
What might you expect for the value of S∘ (entropy) for butane, C4H10?
Entropies provided (Hint: entropy increases with increasing molecular complexity):
- CH4=186.3(J/mol*K)
-C2H6=229.6(J/mol*K)
-C2H8=270.3(J/mol*K)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:10
Amonoprotic acid is an acid that donates a single proton to the solution. suppose you have 0.140 g of a monoprotic acid dissolved in 35.0 ml of water. this solution is then neutralized with 14.5 ml of 0.110 m naoh. what is the molar mass of the acid?
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
Questions

History, 29.09.2019 21:20

Mathematics, 29.09.2019 21:20

History, 29.09.2019 21:20

Chemistry, 29.09.2019 21:20



Mathematics, 29.09.2019 21:20


History, 29.09.2019 21:20

English, 29.09.2019 21:20


English, 29.09.2019 21:20


Mathematics, 29.09.2019 21:20

History, 29.09.2019 21:20


English, 29.09.2019 21:20

History, 29.09.2019 21:20


Mathematics, 29.09.2019 21:20