
Chemistry, 25.03.2020 20:19 ashley232323
Given the balanced equation representing a reaction:
2HCl(aq) + Na2S2O3(aq)--> S(s) + H2SO3(aq) + 2NaCl(aq)
Decreasing the concentration of Na2S2O3(aq) decreases the rate of reaction
because the
(1) activation energy decreases
(2) activation energy increases
(3) frequency of effective collisions decreases
(4) frequency of effective collisions increases

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Given the balanced equation representing a reaction:
2HCl(aq) + Na2S2O3(aq)--> S(s) +...
2HCl(aq) + Na2S2O3(aq)--> S(s) +...
Questions

Law, 27.08.2021 03:30

Mathematics, 27.08.2021 03:30

English, 27.08.2021 03:30

Mathematics, 27.08.2021 03:30


Mathematics, 27.08.2021 03:30



History, 27.08.2021 03:30



Biology, 27.08.2021 03:30


Mathematics, 27.08.2021 03:30

Mathematics, 27.08.2021 03:30

Advanced Placement (AP), 27.08.2021 03:30



English, 27.08.2021 03:30

English, 27.08.2021 03:30

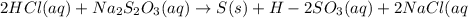
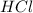 and
and 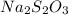 in the given case.
in the given case.

