
Chemistry, 25.03.2020 21:04 dewaynesmith46
Some hydrogen gas is collected over water at 20.0°C. The levels of water inside and outside the gas-collection bottle are the same. The partial pressure of hydrogen is 742.5 torr. What is the barometric pressure at the time the gas is collected?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 23.06.2019 11:00
Just on number 2 (all parts), and if you do answer explain in detail
Answers: 3


Chemistry, 23.06.2019 21:30
Read the chemical equation. n2 + 3h2 → 2nh3 using the volume ratio, determine how many liters of nh3 is produced if 3.6 liters of h2 reacts with an excess of n2, if all measurements are taken at the same temperature and pressure? 5.4 liters 2.4 liters 1.8 liters 1.2 liters
Answers: 1
You know the right answer?
Some hydrogen gas is collected over water at 20.0°C. The levels of water inside and outside the gas-...
Questions


Spanish, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31


Mathematics, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31

English, 19.12.2019 07:31


Physics, 19.12.2019 07:31


Mathematics, 19.12.2019 07:31


History, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31


Mathematics, 19.12.2019 07:31


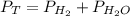
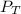 = total pressure
= total pressure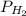 = partial pressure of hydrogen gas = 742.5 mmHg
= partial pressure of hydrogen gas = 742.5 mmHg = partial pressure of water vapor at
= partial pressure of water vapor at  = 17.5 mmHg (standard value)
= 17.5 mmHg (standard value)



