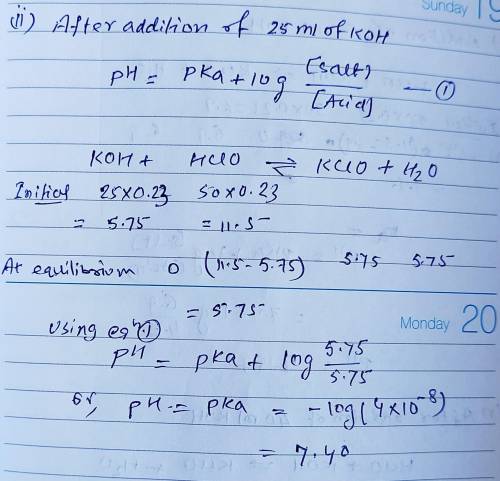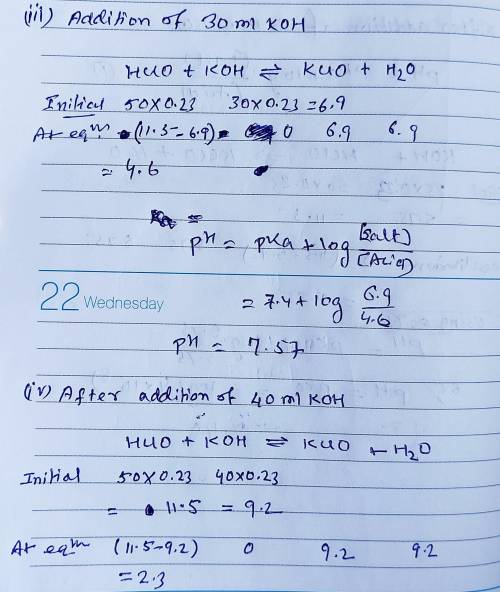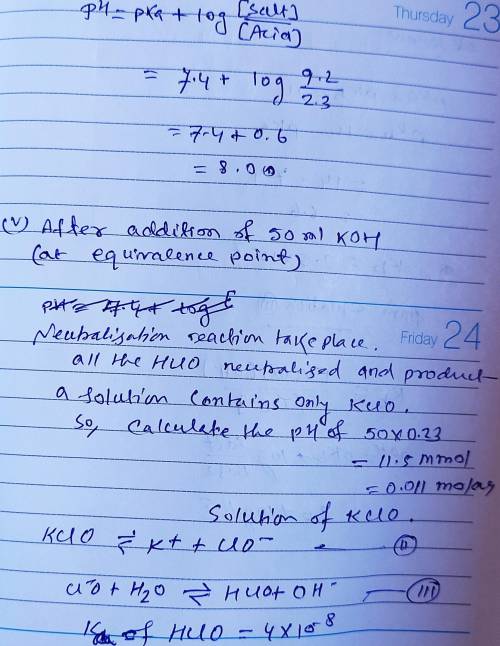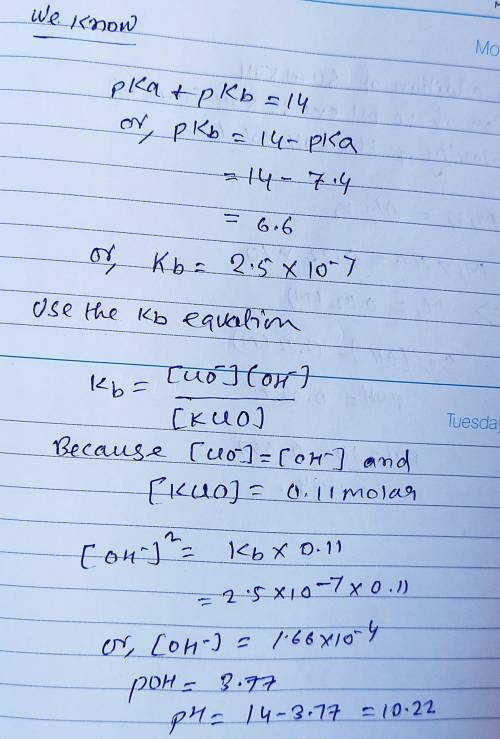Chemistry, 25.03.2020 22:30 eshaesmot12345
Calculate the pH for each case in the titration of 50.0 mL of 0.230 M HClO ( aq ) 0.230 M HClO(aq) with 0.230 M KOH ( aq ) . 0.230 M KOH(aq). Use the ionization constant for HClO . HClO. What is the pH before addition of any KOH ? KOH? pH = pH= What is the pH after addition of 25.0 mL KOH ? 25.0 mL KOH? pH = pH= What is the pH after addition of 30.0 mL KOH ? 30.0 mL KOH? pH = pH= What is the pH after addition of 50.0 mL KOH ? 50.0 mL KOH? pH = pH= What is the pH after addition of 60.0 mL KOH ? 60.0 mL KOH? pH =

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3
You know the right answer?
Calculate the pH for each case in the titration of 50.0 mL of 0.230 M HClO ( aq ) 0.230 M HClO(aq) w...
Questions




Computers and Technology, 26.07.2019 05:30


Health, 26.07.2019 05:30




Arts, 26.07.2019 05:30




History, 26.07.2019 05:30

Health, 26.07.2019 05:30

Arts, 26.07.2019 05:30



English, 26.07.2019 05:30

English, 26.07.2019 05:30