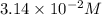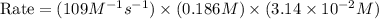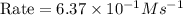Chemistry, 25.03.2020 23:55 markusovaevelyn532
The reaction of nitrogen monoxide with ozone at 25 oC NO + O3NO2 + O2 is first order in NO and first order in O3. Complete the rate law for this reaction in the box below. Use the form k[A]m[B]n... , where '1' is understood for m, n ... (don't enter 1) and concentrations taken to the zero power do not appear. Rate =
In an experiment to determine the rate law, the rate constant was determined to be 109 M-1s-1. Using this value for the rate constant, the rate of the reaction when [NO] = 0.186 M and [O3] = 3.14×10-2 M would be [blank] Ms-1.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2
You know the right answer?
The reaction of nitrogen monoxide with ozone at 25 oC NO + O3NO2 + O2 is first order in NO and first...
Questions

Biology, 12.06.2021 16:40

Biology, 12.06.2021 16:40


Physics, 12.06.2021 16:40

English, 12.06.2021 16:40





Biology, 12.06.2021 16:50

Physics, 12.06.2021 16:50



Mathematics, 12.06.2021 16:50

Mathematics, 12.06.2021 16:50

Mathematics, 12.06.2021 16:50


Mathematics, 12.06.2021 16:50


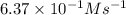
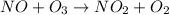
![\text{Rate}=k[NO]^a[O_3]^b](/tpl/images/0564/1543/2796d.png)
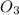 = 1
= 1![\text{Rate}=k[NO]^1[O_3]^1](/tpl/images/0564/1543/85a0b.png)
![\text{Rate}=k[NO][O_3]](/tpl/images/0564/1543/1a568.png)
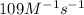
![[O_3]](/tpl/images/0564/1543/8b13e.png) = concentration of
= concentration of