

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1


Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
The rate constant for the reaction is 0.660 M − 1 ⋅ s − 1 0.660 M−1⋅s−1 at 200 ∘ C. 200 ∘C. A ⟶ prod...
Questions

Mathematics, 17.11.2020 05:00

History, 17.11.2020 05:00

History, 17.11.2020 05:00


Mathematics, 17.11.2020 05:00





Mathematics, 17.11.2020 05:00

Mathematics, 17.11.2020 05:00


Mathematics, 17.11.2020 05:00



Chemistry, 17.11.2020 05:00

Spanish, 17.11.2020 05:00

Advanced Placement (AP), 17.11.2020 05:00

Biology, 17.11.2020 05:00

Mathematics, 17.11.2020 05:00

![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0564/2226/5ea71.png)
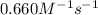
![[A]_o](/tpl/images/0564/2226/9caf5.png) = Initial concentration = 0.00440 M
= Initial concentration = 0.00440 M![0.660=\frac{1}{865}\left (\frac{1}{[A]}-\frac{1}{(0.00440)}\right)](/tpl/images/0564/2226/9d3b6.png)
![[A]=0.00125M](/tpl/images/0564/2226/63ab7.png)


