

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1

Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1
You know the right answer?
The first-order isomerization reaction: cyclopropane → propene has a rate constant of 1.10×10-4 s-1...
Questions



Mathematics, 31.07.2019 11:40

Physics, 31.07.2019 11:40



Mathematics, 31.07.2019 11:40


English, 31.07.2019 11:40



Mathematics, 31.07.2019 11:40

Chemistry, 31.07.2019 11:40

Mathematics, 31.07.2019 11:40


Business, 31.07.2019 11:40




Chemistry, 31.07.2019 11:40

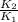 =
= 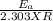
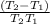
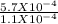 =
= 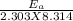
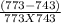
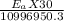
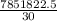 j/mole = 261.7 Kj/mole
j/mole = 261.7 Kj/mole

