
At 400 k, the equilibrium constant for the reaction br2 (g) + cl2 (g) 2brcl (g) is kp = 7.0. a closed vessel at 400 k is charged with 1.00 atm of br2 (g), 1.00 atm of cl2 (g), and 2.00 atm of brcl (g). use q to determine which of the statements below is true.
a. the equilibrium partial pressure of br2 will be greater than 1.00 atm.
b. the reaction will go to completion since there are equal amounts of br2 and cl2.
c. the equilibrium partial pressure of brcl (g) will be greater than 2.00 atm.
d. the equilibrium partial pressures of br2, cl2, and brcl will be the same as the initial values.
e. at equilibrium, the total pressure in the vessel will be less than the initial total pressure.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
At 400 k, the equilibrium constant for the reaction br2 (g) + cl2 (g) 2brcl (g) is kp = 7.0. a close...
Questions

History, 07.07.2019 20:30

Mathematics, 07.07.2019 20:30

Mathematics, 07.07.2019 20:30

Social Studies, 07.07.2019 20:30


Mathematics, 07.07.2019 20:30








History, 07.07.2019 20:30


Physics, 07.07.2019 20:30

Mathematics, 07.07.2019 20:30



English, 07.07.2019 20:30

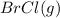 will be greater than
will be greater than 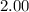 atm.
atm.![Q_{p} =\frac{[products]}{[reactants]}](/tpl/images/0564/6775/77b1b.png)
![Q_{p} = \frac{[2.00]^2}{{[1.00]}[1.00]}](/tpl/images/0564/6775/b1f67.png)
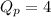
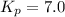
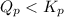 .
.

