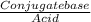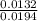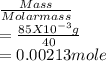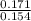Chemistry, 26.03.2020 05:19 kkjones1536
A 100.0-mL buffer solution is 0.175 M in HClO and 0.150 M in NaClO. Part A: What is the initial pH of this solution?Part B: What is the pH after addition of 150.0 mg of HBr?Part C: What is the pH after addition of 85.0 mg of NaOH?

Answers: 2
Another question on Chemistry



Chemistry, 23.06.2019 06:40
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
A 100.0-mL buffer solution is 0.175 M in HClO and 0.150 M in NaClO. Part A: What is the initial pH o...
Questions





History, 22.07.2019 17:10

Biology, 22.07.2019 17:10

Chemistry, 22.07.2019 17:20

Geography, 22.07.2019 17:20

Biology, 22.07.2019 17:20

History, 22.07.2019 17:20

Mathematics, 22.07.2019 17:20


Mathematics, 22.07.2019 17:20







![\frac{[Conjugate base]}{[Acid]}](/tpl/images/0564/8323/225c1.png)
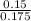
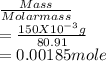
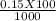 = 0.015
= 0.015 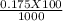 = 0.0175
= 0.0175