

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
How many moles of fe2o3 will be produced from 18.0 g of fe assuming o2 is available in excess...
Questions


English, 14.07.2020 02:01


Mathematics, 14.07.2020 02:01





Arts, 14.07.2020 02:01

English, 14.07.2020 02:01


Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01


Mathematics, 14.07.2020 02:01

Social Studies, 14.07.2020 02:01


Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

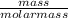
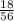 = 0.32mole
= 0.32mole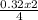 = 0.161 moles of Fe₂O₃
= 0.161 moles of Fe₂O₃


