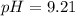Chemistry, 26.03.2020 17:49 seaotter9630
A chemist titrates 90.0 mL of a 0.5870 M acetic acid (HCH3CO2) solution with 0.4794M NaOH solution at 25 °C. Calculate the pH at equivalence. The p Kg of acetic acid is 4.76. Round your answer to 2 decimal places.

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 05:00
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1

Chemistry, 23.06.2019 13:30
How many ammonium ions and how many sulfate ions are present in a 0.270 mol sample of ?
Answers: 1

You know the right answer?
A chemist titrates 90.0 mL of a 0.5870 M acetic acid (HCH3CO2) solution with 0.4794M NaOH solution a...
Questions

Mathematics, 02.10.2020 16:01

Mathematics, 02.10.2020 16:01

Mathematics, 02.10.2020 16:01

Spanish, 02.10.2020 16:01



Mathematics, 02.10.2020 16:01

Computers and Technology, 02.10.2020 16:01


Geography, 02.10.2020 16:01

English, 02.10.2020 16:01



History, 02.10.2020 16:01

Chemistry, 02.10.2020 16:01


Health, 02.10.2020 16:01



Mathematics, 02.10.2020 16:01

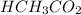 = 0.5870 M
= 0.5870 M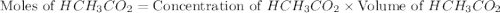
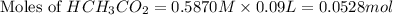
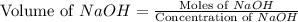
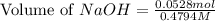
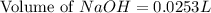
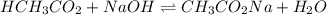
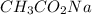 = 0.0528 mol
= 0.0528 mol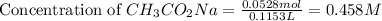
![pH=\frac{1}{2}[pK_w+pK_a+\log C]](/tpl/images/0565/3060/b44e5.png)
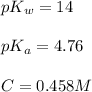
![pH=\frac{1}{2}[14+4.76+\log (0.458)]](/tpl/images/0565/3060/e66f0.png)