
Chemistry, 26.03.2020 18:46 briannabrewer123
When a reaction has reached equilibrium... A. Q = K B. The rate of the forward reaction and the rate of the reverse reaction are equal. C. Both the forward and reverse reactions stop occurring. D. The concentration of products and the concentration of reactants are equal. E. The forward rate constant and the reverse rate constant are equal.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
When a reaction has reached equilibrium... A. Q = K B. The rate of the forward reaction and the rate...
Questions


Business, 11.03.2020 02:13





Computers and Technology, 11.03.2020 02:13


Mathematics, 11.03.2020 02:13

Mathematics, 11.03.2020 02:13

Mathematics, 11.03.2020 02:13





Biology, 11.03.2020 02:14


Mathematics, 11.03.2020 02:14


 .
.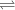 cC
cC![K_{eq}=\frac{[C]^c}{[A]^a[B]^b}](/tpl/images/0565/3634/98de9.png)


