
For the reactions system 2H2(g) + S2(g) 2H2S(g), a 1.00-liter vessel is found to contain 0.5 mole of H2 , 0.02 mole of S2, and 68.5 moles of H2S. What is the equilibrium constant expression? What are the chemical formulas, not numbers. (Enter subscripts after the letters: for example, H2O = Hs2O. Also, don't forget to use the proper chemical shorthand for chemical symbols. For instance, chlorine = Cl but not cl or cL.) Please and thank you!! :) NOT IN Numbers
This Image is how I have to answer it
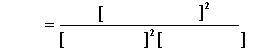

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2


Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2
You know the right answer?
For the reactions system 2H2(g) + S2(g) 2H2S(g), a 1.00-liter vessel is found to contain 0.5 mole of...
Questions

Computers and Technology, 14.04.2020 22:16





History, 14.04.2020 22:16


History, 14.04.2020 22:16












Mathematics, 14.04.2020 22:16



