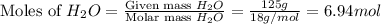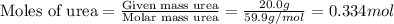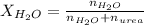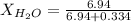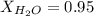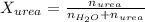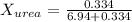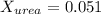Chemistry, 26.03.2020 19:59 nerikzagallegos
. You prepare a solution by adding 20.0 g Urea [(NH2)2CO] to 125 g water at 25.0oC & vapor pressure 23.76 torr. The solution vapor pressure is 22.67 torr. Calculate the molecular weight of urea. XH2O = moles H2O = Xsolute = moles urea = molar mass = calculations:

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
. You prepare a solution by adding 20.0 g Urea [(NH2)2CO] to 125 g water at 25.0oC & vapor press...
Questions

History, 22.10.2019 17:50

Mathematics, 22.10.2019 17:50

Mathematics, 22.10.2019 17:50


History, 22.10.2019 17:50

English, 22.10.2019 17:50

Physics, 22.10.2019 17:50


Mathematics, 22.10.2019 17:50

Mathematics, 22.10.2019 17:50

Mathematics, 22.10.2019 17:50


Mathematics, 22.10.2019 17:50



Mathematics, 22.10.2019 17:50


Mathematics, 22.10.2019 17:50


Computers and Technology, 22.10.2019 17:50

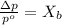
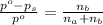
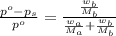
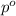 = vapor pressure of the pure solvent water = 23.76 torr
= vapor pressure of the pure solvent water = 23.76 torr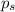 = vapor pressure of the solution = 22.67 torr
= vapor pressure of the solution = 22.67 torr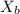 = mole fraction of solute (urea)
= mole fraction of solute (urea)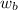 = mass of urea = 20.0 g
= mass of urea = 20.0 g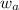 = mass of water = 125 g
= mass of water = 125 g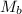 = molar mass of urea = ?
= molar mass of urea = ?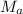 = molar mass of water = 18 g/mol
= molar mass of water = 18 g/mol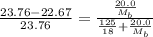
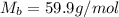
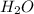 and urea.
and urea.