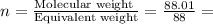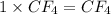Chemistry, 26.03.2020 20:01 nhester3401
A compound is found to contain 13.65 % carbon and 86.35 % fluorine by mass. To answer the question, enter the elements in the order presented above. QUESTION 1: The empirical formula for this compound is . QUESTION 2: The molar mass for this compound is 88.01 g/mol. The molecular formula for this compound is .

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1

Chemistry, 23.06.2019 05:20
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2
You know the right answer?
A compound is found to contain 13.65 % carbon and 86.35 % fluorine by mass. To answer the question,...
Questions


Biology, 28.07.2021 09:50

Mathematics, 28.07.2021 09:50


History, 28.07.2021 09:50

English, 28.07.2021 09:50



Business, 28.07.2021 09:50

Chemistry, 28.07.2021 09:50


Mathematics, 28.07.2021 09:50




History, 28.07.2021 09:50

Physics, 28.07.2021 09:50

Mathematics, 28.07.2021 09:50


Computers and Technology, 28.07.2021 09:50

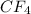
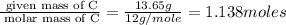
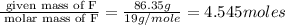
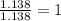
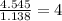
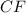 = 1(12)+4(19)= 88g.
= 1(12)+4(19)= 88g.