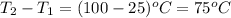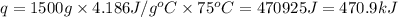How much heat is required to warm 1.50L of water from 25.0C to 100.0C? (Assume a density of 1.0g/mL for the water.)
My brain wants to just start with 1.5L and use density as a conversion factor but I seriously think I'm missing something. I don't really understand the heat equation, but I'm thinking I might need to use q = mCAT?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
How much heat is required to warm 1.50L of water from 25.0C to 100.0C? (Assume a density of 1.0g/mL...
Questions

Mathematics, 25.12.2021 15:40


SAT, 25.12.2021 15:50

Advanced Placement (AP), 25.12.2021 15:50



World Languages, 25.12.2021 15:50


Biology, 25.12.2021 15:50




Physics, 25.12.2021 15:50

Mathematics, 25.12.2021 15:50



Physics, 25.12.2021 15:50


World Languages, 25.12.2021 16:00

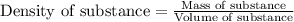
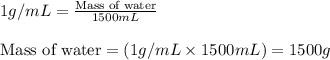
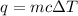
 = change in temperature =
= change in temperature =