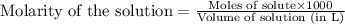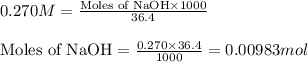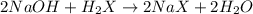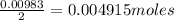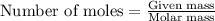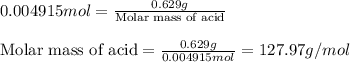A 0.629 g sample of a diprotic acid is dissolved in water and titrated with 0.270 M NaOH. What is the molar mass of the acid if 36.4 mL of the NaOH solution is required to neutralize the sample? Assume the volume of NaOH corresponds to the second equivalence point. A flask with a solution sits on the base of a ring stand. A buret filled with liquid is suspended above the flask by the ring stand. molar mass: g/mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
A 0.629 g sample of a diprotic acid is dissolved in water and titrated with 0.270 M NaOH. What is th...
Questions

Biology, 24.09.2019 23:30





Biology, 24.09.2019 23:30

Spanish, 24.09.2019 23:30



English, 24.09.2019 23:30

Mathematics, 24.09.2019 23:30


History, 24.09.2019 23:30

History, 24.09.2019 23:30




History, 24.09.2019 23:30

Social Studies, 24.09.2019 23:30

English, 24.09.2019 23:30