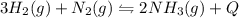In the reaction represented by the equation n2(g) + 3h2(g) 2nh3(g), the
forward reaction is ex...

Chemistry, 10.10.2019 16:30 jacobbecker99
In the reaction represented by the equation n2(g) + 3h2(g) 2nh3(g), the
forward reaction is exothermic. which set of conditions would give the best yield of
ammonia at equilibrium?
a) 800 atmospheres and 2000 oc
b) 1 atmosphere and 500 o
c
c) 1 atmosphere and 2000 o
c
d) 800 atmospheres and 500 o
c

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1
You know the right answer?
Questions




Mathematics, 21.08.2019 20:00




Geography, 21.08.2019 20:00


French, 21.08.2019 20:00



Arts, 21.08.2019 20:00

Mathematics, 21.08.2019 20:00



Health, 21.08.2019 20:00

Mathematics, 21.08.2019 20:00


Mathematics, 21.08.2019 20:00