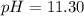Chemistry, 26.03.2020 20:36 gennhill14
A chemist titrates 100.0mL of a 0.3444M hydrocyanic acid HCN solution with 0.8414M KOH solution at 25°C . Calculate the pH at equivalence. The pKa of hydrocyanic acid is 9.21 . Round your answer to 2 decimal places.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1


Chemistry, 23.06.2019 14:00
If you fill your car tire to a pressure of 32 psi (pounds per square inch) on a hot summer day when the temperature is 35°c (95°f), what is the pressure (in psi) on a cold winter day when the temperature is -15°c (5°f)? assume no gas leaks out between measurements and the volume of the tire does not change.
Answers: 1
You know the right answer?
A chemist titrates 100.0mL of a 0.3444M hydrocyanic acid HCN solution with 0.8414M KOH solution at 2...
Questions


Chemistry, 03.08.2019 06:00


Biology, 03.08.2019 06:00

Computers and Technology, 03.08.2019 06:00

Biology, 03.08.2019 06:00

Computers and Technology, 03.08.2019 06:00


Computers and Technology, 03.08.2019 06:00

History, 03.08.2019 06:00

Computers and Technology, 03.08.2019 06:00

Mathematics, 03.08.2019 06:00

History, 03.08.2019 06:00

Biology, 03.08.2019 06:00


Mathematics, 03.08.2019 06:00




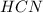 = 0.3444 M
= 0.3444 M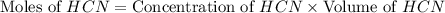
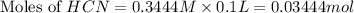
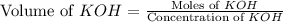
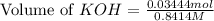
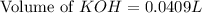
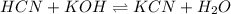
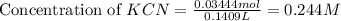
![pH=\frac{1}{2}[pK_w+pK_a+\log C]](/tpl/images/0565/6867/b44e5.png)
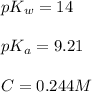
![pH=\frac{1}{2}[14+9.21+\log (0.244)]](/tpl/images/0565/6867/e6178.png)