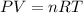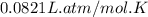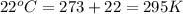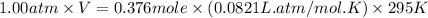Chemistry, 26.03.2020 21:07 cbyearty56361
Automobile airbags contain solid sodium azide, NaN3, that reacts to produce nitrogen gas when heated, thus inflating the bag. 2NaN3(s)⟶2Na(s)+3N2(g) Calculate the value of work, w, for the system if 16.3 g NaN3 reacts completely at 1.00 atm and 22 ∘ C. w=

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1

Chemistry, 23.06.2019 15:40
Glucose (c6h12o6) is the simple sugar that plants make. what is the total number of atoms in glucose? 1 3 24 144
Answers: 1
You know the right answer?
Automobile airbags contain solid sodium azide, NaN3, that reacts to produce nitrogen gas when heated...
Questions


Mathematics, 02.03.2021 19:50

Social Studies, 02.03.2021 19:50

Mathematics, 02.03.2021 19:50

Mathematics, 02.03.2021 19:50

Mathematics, 02.03.2021 19:50


History, 02.03.2021 19:50


Biology, 02.03.2021 19:50



Mathematics, 02.03.2021 19:50

Physics, 02.03.2021 19:50



Mathematics, 02.03.2021 19:50

Mathematics, 02.03.2021 19:50

Mathematics, 02.03.2021 19:50

Mathematics, 02.03.2021 19:50

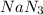
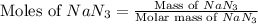
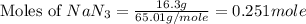
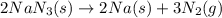
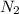
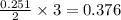 moles of
moles of