
Chemistry, 26.03.2020 22:01 kharmaculpepper
Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the rate of disappearance of Cl2 is 4.44 × 10-2 M/s, what is the rate of disappearance of NO? 2 NO(g) + Cl2(g) → 2 NOCl(g) Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the rate of disappearance of Cl2 is 4.44 × 10-2 M/s, what is the rate of disappearance of NO? 2 NO(g) + Cl2(g) → 2 NOCl(g) 2.22 × 10-2 M/s 1.11 × 10-1 M/s 4.44 × 10-2 M/s 8.88 × 10-2 M/s 5.25 × 10-2 M/s

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 13:30
What would happen if no were added to n(g)+o2=2no(g) at equilibrium?
Answers: 1
You know the right answer?
Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the...
Questions




Computers and Technology, 24.06.2021 22:50

Computers and Technology, 24.06.2021 22:50




Mathematics, 24.06.2021 22:50

Mathematics, 24.06.2021 22:50

Mathematics, 24.06.2021 22:50

History, 24.06.2021 22:50



Mathematics, 24.06.2021 22:50




Computers and Technology, 24.06.2021 22:50

SAT, 24.06.2021 22:50

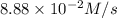
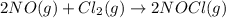
![-\frac{1d[NO]}{2dt}=-\frac{d[Cl_2]}{dt}=+\frac{1d[NOCl]}{2dt}](/tpl/images/0565/9272/04d4d.png)
![\frac{-d[Cl_2]}{dt}]=4.44\times 10^{-2}M/s](/tpl/images/0565/9272/271c7.png)
![-\frac{1d[NO]}{dt}=2\times {\frac{-d[Cl_2]}{dt}=2\times 4.44\times 10^{-2}M/s=8.88\times 10^{-2}M/s](/tpl/images/0565/9272/dea34.png)


