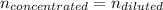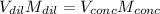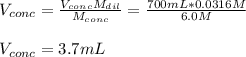Chemistry, 26.03.2020 21:52 MathChic68
A chemist must prepare 700.0mL of nitric acid solution with a pH of 1.50 at 25°C. He will do this in three steps: Fill a 700.0mL volumetric flask about halfway with distilled water. Measure out a small volume of concentrated (6.0M) stock nitric acid solution and add it to the flask. Fill the flask to the mark with distilled water. Calculate the volume of concentrated nitric acid that the chemist must measure out in the second step. Round your answer to 2 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 21.06.2019 22:50
Achemical reaction (also known as a chemical change) produces substances that are chemically different from the starting materials. an example of a chemical reaction is the formation of water from hydrogen and oxygen gas.in a physical change, a substance changes its physical appearance but not its chemical identity. an example of physical change is the formation of liquid water from solid water, a familiar process called melting. physically, liquid water looks very different from solid water (ice) but the chemical identity, water, is the same for both. which of following changes that affect the composition of our atmosphere involve physical changes and which involve chemical reactions? oxygen gas changes to ozone during thunderstorms carbon dioxide is produced by the combustion of gasoline in an automobile engine. when coal, oil, and natural gas are decomposed in landsills they produce methane gas. freezing rain develops when a warm air mass overrides a cold air mass. fog forms from water vapor when the temperature drops below the dew point
Answers: 1


Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1
You know the right answer?
A chemist must prepare 700.0mL of nitric acid solution with a pH of 1.50 at 25°C. He will do this in...
Questions





Physics, 30.10.2020 04:50

Mathematics, 30.10.2020 04:50



Mathematics, 30.10.2020 04:50


Health, 30.10.2020 04:50



Physics, 30.10.2020 04:50


Mathematics, 30.10.2020 04:50



Mathematics, 30.10.2020 04:50

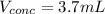
![[H]^+=10^{-pH}=10^{-1.50}=0.0316M](/tpl/images/0565/8791/85748.png)
![[H]^+=[HNO_3]=0.0316M](/tpl/images/0565/8791/de553.png)