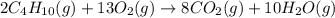Chemistry, 26.03.2020 22:43 genyjoannerubiera
Combustion of hydrocarbons such as butane (C4H10) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason, there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.
Write a balanced chemical equation, including physical state symbols, for the combustion of gaseous butane into gaseous carbon dioxide and gaseous water.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
Combustion of hydrocarbons such as butane (C4H10) produces carbon dioxide, a "greenhouse gas." Green...
Questions

Mathematics, 28.09.2019 10:10





Mathematics, 28.09.2019 10:10



Mathematics, 28.09.2019 10:10

Mathematics, 28.09.2019 10:10

Computers and Technology, 28.09.2019 10:10

Mathematics, 28.09.2019 10:10

Biology, 28.09.2019 10:10

Biology, 28.09.2019 10:10

History, 28.09.2019 10:10


Mathematics, 28.09.2019 10:10


Geography, 28.09.2019 10:10

History, 28.09.2019 10:10