
Chemistry, 26.03.2020 22:48 giraffesaur44
Determine the expression for the rate of the reaction with respect to each of the reactants and products. Determine the expression for the rate of the reaction with respect to each of the reactants and products. Rate=−13Δ[A]Δt=−Δ[B]Δt=12Δ[C]ΔtRate =−13Δ[A]Δt=−Δ[B]Δt=12Δ[C]Δt Rate=−12Δ[A]Δt=−Δ[B]Δt=13Δ[C]ΔtRate =−12Δ[A]Δt=−Δ[B]Δt=13Δ[C]Δt Rate=−Δ[A]Δt=−12Δ[B]Δt=13Δ[C]ΔtRate =−Δ[A]Δt=−12Δ[B]Δt=13Δ[C]Δt Rate=12Δ[A]Δt=12Δ[B]Δt=13Δ[C]Δt

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3



Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
Determine the expression for the rate of the reaction with respect to each of the reactants and prod...
Questions




Mathematics, 26.02.2021 23:50


Mathematics, 26.02.2021 23:50

Mathematics, 26.02.2021 23:50





Mathematics, 26.02.2021 23:50


History, 26.02.2021 23:50



History, 26.02.2021 23:50



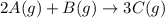
![Rate=-\frac{1}{3}\frac{\Delta [A]}{\Delta t}=-\frac{\Delta [B]}{\Delta t}=\frac{1}{2}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/ff0f8.png)
![Rate=-\frac{1}{2}\frac{\Delta [A]}{\Delta t}=-\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/11b91.png)
![Rate=-\frac{\Delta [A]}{\Delta t}=-\frac{1}{2}\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/f7a0b.png)
![Rate=\frac{1}{2}\frac{\Delta [A]}{\Delta t}=\frac{1}{2}\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/3152a.png)
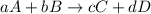
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0566/1120/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0566/1120/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0566/1120/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0566/1120/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0566/1120/d4b94.png)
![\text{Rate of disappearance of }A=-\frac{1}{2}\frac{\Delta [A]}{\Delta t}](/tpl/images/0566/1120/4cd85.png)
![\text{Rate of disappearance of }B=-\frac{\Delta [B]}{\Delta t}](/tpl/images/0566/1120/43240.png)
![\text{Rate of formation of }C=+\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/d1447.png)


